Background: Standard therapies for patients with newly diagnosed multiple myeloma (NDMM) who are ineligible for autologous stem cell transplantation (ASCT) have been lenalidomide-dexamethasone (Rd), bortezomib-lenalidomide-dexamethasone (VRd) or bortezomib-melphalan-prednisone (VMP). The benefit of adding an anti-CD38 monoclonal antibody (mAb) to the standard treatments of VMP and Rd has been demonstrated in two large phase 3 studies, achieving overall response rates (ORR) of 90.9% and 92.9% in the daratumumab-VMP arm and the daratumumab-Rd arm, respectively (Facon, 2019, Mateos, 2018). Isatuximab is an anti-CD38 mAb approved in combination with pomalidomide-dexamethasone and carfilzomib-dexamethasone to treat relapsed/refractory MM. The drug has also shown clinical responses as combination therapy to treat NDMM (Trudel, 2019).
Corticosteroids have been the backbone of most myeloma-targeted therapies since the discovery of their effectiveness. Due to the continuous treatment paradigm, the cumulative dose of corticosteroids in patients is substantial. Steroids inflict reduction in both short- and long-term quality of life and increase patients' receptiveness to infections. Infections are one of the main reasons for complications and death during active first line treatment and the rate rises with age (Mothy, 2019).
Instead of reducing or removing corticosteroids, isatuximab will replace corticosteroids to evaluate the effectiveness and safety of the novel regime of isatuximab-bortezomib-lenalidomide.
Aim: The study will evaluate isatuximab (Isa) in combination with bortezomib (V) and lenalidomide (R) with minimal dexamethasone (d) as first-line treatment in transplant-ineligible patients. The primary endpoint is the number of patients who achieve measurable residual disease negative (Euroflow NGF 10 -5) complete response during and/or after 18 cycles of study treatment. Secondary endpoints include progression free survival, overall survival, overall response rate, safety evaluations and patient-reported outcome.
Methods: The REST study is an academic, single arm, open-label, phase 2 study of NDMM patients ineligible for ASCT. 51 patients are included and receive Isa-VRd (Isa: 10 mg/kg IV Days 1, 8, 15, 22 during cycle 1, Q2W cycle 2-18; V: 1, 3 mg/m 2 SC Days 1, 8, 15 during cycle 1-8; R: 25 mg PO Days 1-21 during all cycles; d: 20 mg PO Days 1, 8, 15, 22 only for the first 2 cycles), all 28-day cycles.
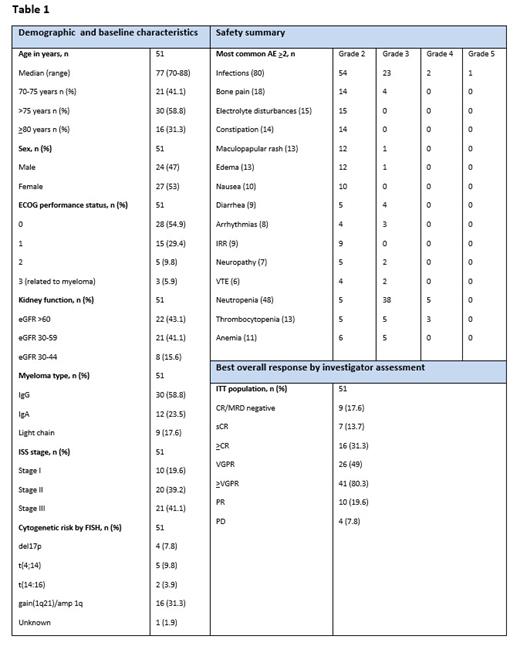
Results: Recruitment was completed in January 2023. Baseline characteristics, safety summary and preliminary results can be found in Table 1. The median age is 77 years, range 70-88 years. Twenty-nine patients developed 60 grade >3 non-hematological adverse events (AE), including infections (n=26), syncope (n=5), diarrhea (n=4), skeletal pain (n=4), arrhythmias (n=3), acute renal failure (n=3), increased transaminases (n=3), venous thromboembolism (n=2), arthritis (n=2), gastrointestinal hemorrhage (n=2), peripheral sensory neuropathy (n=2), edema (n=1), hyperglycemia (n=1), rash (n=1) and opiate intoxication (n=1).
At a median follow up of 12 months, the ORR was 100% (51/51) with very good partial response or better at80.3% (41/51). Forty-four patients are still on study. One patient died from septicemia with staphylococcus aureus, four patients discontinued due to disease progression, one patient due to poor compliance and one patients for safety reasons judged by the investigator.
Conclusions: Isa-VRd in the transplant ineligible population with a median age of 77 years has a tolerable safety profile. Isa-VRd is showing encouraging preliminary efficacy in NDMM ineligible for transplant. Follow-up is ongoing.
Acknowledgements: Sanofi funded this research.
Disclosures
Askeland:Amgen: Honoraria; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Slørdahl:GSK: Consultancy; Bristol Myers Squibb: Consultancy; Janssen-Cilag: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Honoraria. Hermansen:Amgen: Honoraria; Janssen-Cilag: Honoraria, Research Funding; Bristol Myers Scuibb: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Takeda: Honoraria; Pfizer: Honoraria; Roche: Honoraria. Schjesvold:Skylite DX: Other: Honoraria for lectures and educational material; Celgene: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Takeda: Consultancy, Other: Honoraria for lectures and educational material; Oncopeptides: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Janssen-Cilag: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Sanofi: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Bristol Myers Squibb: Consultancy, Other: Honoraria for lectures and educational material; Targovax: Research Funding; Amgen: Other: Honoraria for lectures and educational material; Novartis: Other: Honoraria for lectures and educational material; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Pfizer: Other: Honoraria for lectures and educational material; Abbvie: Consultancy, Other: Honoraria for lectures and educational material; Daiichi Sankyo: Other: Honoraria for lectures and educational material; Schain: Other: Honoraria for lectures and educational material.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal